
copies of Ionic versus Covalent Bonding worksheet ( S-C-6-1_Ionic versus Covalent Bonding and KEY.doc).
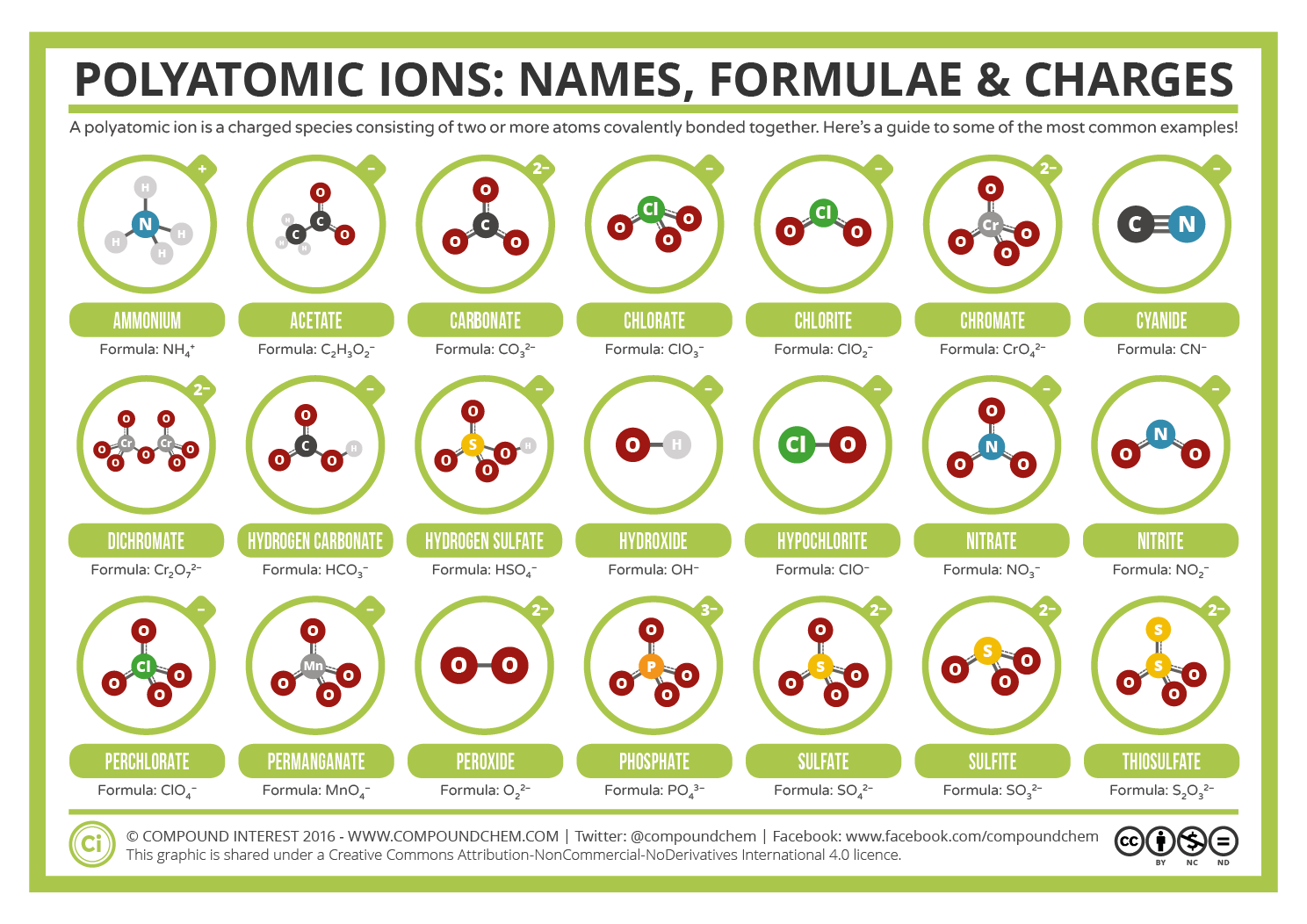
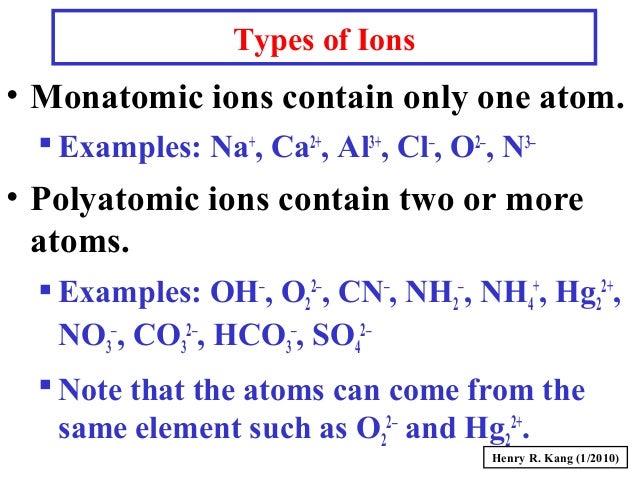
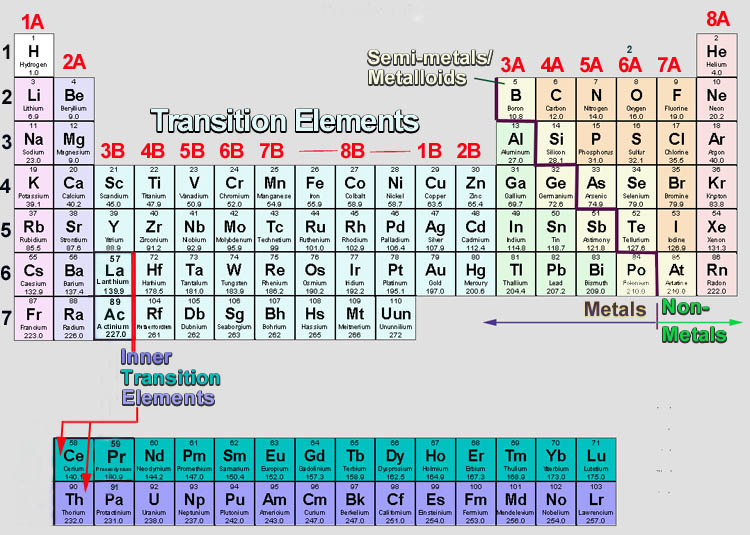
copies of Covalent Bonding worksheet ( S-C-6-1_Covalent Bonding and KEY.doc).copies of Ionic Bonding worksheet ( S-C-6-1_Ionic Bonding and KEY.doc).Covalent and Ionic Bonds ( S-C-6-1_Covalent and Ionic Bonds.doc).Electronegativity Trends in the Periodic Table ( S-C-6-1_Electronegativity Trends.doc).Octet Rule Chart ( S-C-6-1_Octet Rule Chart and KEY.doc).copies of Valence Electrons and Lewis Dot Structures handout S-C-6-1_Valence Electrons and Lewis Dot Structures and KEY.doc).Periodic Table of the Elements ( S-C-6-1_Periodic Table.pdf).Valence Electron: Electrons in the outermost energy level of an atom Electrons that can be involved in chemical bonding.Octet Rule (Noble Gas Rule): Rule that states that atoms tend to combine in such a way that they each have eight electrons in the highest main energy level, giving them the same electronic configuration as a noble gas.Neutral Atom: An atom with equal numbers of protons and electrons.Molecule: A unit of matter formed by the chemical bonding of two or more atoms by covalent bonds.Also used to represent bonding between atoms. Lewis Dot Structure: A diagram to represent the valence electrons of an atom, written as the element symbol surrounded by dots that represent the valence electrons.Ionic Compound: A compound formed by ions.Ionic Bond: Bond formed between oppositely charged ions because of electrostatic forces of attraction.Ion: An atom that has acquired an electrical charge by either gaining or losing electrons.Electronegativity: A measure of the attraction of an atom for the electrons in a covalent bond.Dipole: The uneven distribution of electrical charge across a polar molecule.Covalent Bond: A chemical bond formed by the sharing of electrons between atoms.Compound: A substance formed by the chemical combination of elements in defined proportions.Cation: An ion that has a positive charge.Atoms can exist alone or in combinations with other atoms forming molecules. Atom: The smallest unit of an element that retains the chemical properties of the element.Anion: An ion that has a negative charge.


 0 kommentar(er)
0 kommentar(er)
